TEK NET
The Henderson Amateur Radio Club is pleased to present our Tek Net every Sunday evening, starting at 8pm local time (0300 UTC).
The audio portion of the Tek Net will be found on the Henderson Amateur Radio Club Repeater Network (RF). The audio may also be accessed via Echolink (W7HEN-R node 740644) and Allstar (node 44045).
The audio will refer to this page, so that the listener can more fully understand the concepts as they are presented.
All are welcome!
October 16, 2022
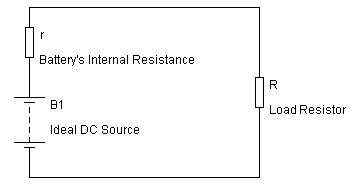
“Internal resistance in batteries and the it’s effect.”
Some of you may have heard about ‘internal resistance’ in a battery, but what is it really?
How can a battery have a resistor inside it?
The answer to that question can be simple…and a bit complex also.
There are DC methods of measuring the Internal Resistance and AC method of measuring the impedence of a battery…today we are talking about DC method only.
Internal resistance (often called the “GATE KEEPER”) of a battery is technically defined as ‘the opposition of current flow within the battery cell’.
There are two types of resistances involved in the internal resistance, they are ionic resistance and electronic resistance. (explain if interested)
Ionic resistance occurs from various electrochemical factors like electrolyte conductivity, ion mobility, and the electrode surface area.
The electronic resistance refers to the actual electrical resistance of the battery itself. This value determines how much energy (electricity) is burned up as heat when the battery is under load ( current flowing into or out of the battery), now you know why they get warm…
How to find the Internal Resistance…
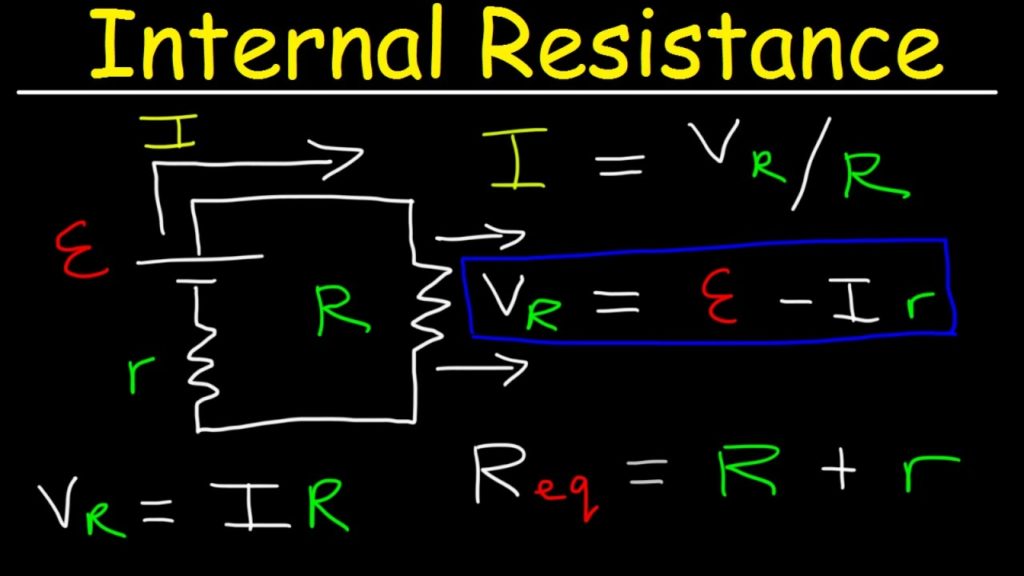
The most common method for determining a battery’s internal resistance is to:
– connect the battery to a load with a resistor,
– measure voltage through the battery,
– calculate current,
– measure voltage through the resistor, find the voltage drop,
– using Kirchhoff’s law to determine the remaining resistance, which will be the internal resistance.
Internal resistance is affected by a variety of factors, including state of charge (SOC), temperature, electrolyte, separator, anode electrode, cathode electrode, and battery size,
but for today’s discussion we aren’t going to include all of these factors.
I guess an easy way to say it would be that volts are lost in the internal resistance of the battery cell, the more current that is drawn from the battery, the more volts are lost….thus providing less voltage for your circuit needs.